
It is a chemical change because a new element is produced.Ģ0- When an egg is cooked, it goes through a chemical change.Ģ1- When mixing sodium bicarbonate With vinegar, carbon dioxide is produced.Ģ2- When meat is roasted on a grill, an irreversible chemical change is given.Ģ3- The refining of the oil to produce gasoline generates a new substance, and therefore, is a chemical change.Ģ4- The Sodium hydroxide (NaOH) is combined with hydrochloric acid (HCl) to form sodium chloride (NaCl), also known as common salt.Ģ5- The creation of wine from grapes is a process of fermentation. This bubbling means that the carbonic acid in the beverage has been broken, releasing the carbon dioxide.ġ8- Steel is an alloy between iron and other elements, mainly carbon. ġ3 - When a match is lit, a chemical change occurs.ġ4- The burning of natural gas, like the methane gas used by some kitchens, is a chemical change.ġ5- When a piece of cheese or bread has mold, it is a sign that a chemical change has occurred.ġ6- When a photo is revealed, a photochemical reaction occurs.ġ7- When you open a bottle of champagne or some soft drink, you will see a bubbling. For example, when burning wood in a chimney produces a new substance, the ashes, and it is an irreversible reaction.ĩ- When the fruit matures, as well as when it rots, chemical changes generated by the hormones take place.ġ0- The oxidation of the metals that are left to the interperie is a chemical reaction between the metal and the humidity in the air.ġ1- Photosynthesis, the process carried out by plants to obtain glucose, is a chemical change.ġ2- Yogurt is the result of a chemical change involving milk and certain bacteria, such as Streptococcus thermophilus and the Lactobacillus bulgaricus. For example, rotten eggs go through a process of decomposition that causes them to change color and smell.ħ- The process of digestion in humans and animals is also a chemical change.Ĩ- Combustion is a chemical change. This is called oxidation.Ĥ- When baking a cake, this mixture absorbs the heat (endothermic reaction) and goes from being raw to cooked.ĥ- A camphor passes from solid to gaseous state and can not be returned to its initial state, indicating that a chemical change occurred.Ħ- When the food rots, a chemical change occurs. With temperature changes in autumn and winter, plants stop the process of photosynthesis, thus stopping the synthesis of chlorophyll Therefore, the leaves of the plant take the color of the other available pigments: yellow and red.ģ- When you pick an apple and leave it outdoors, it changes from being ivory to brown or ocher. In addition to chlorophyll, plants have other pigments in their leaves: carotenoids and anthocyanins, which are yellow and red respectively. The green color of the leaves is due to the presence of chlorophyll This substance is necessary for the photosynthesis, So the plant must synthesize continuously.

30 examples of chemical changesġ- The explosion of the fireworks is an example of chemical change.Ģ- The change of color in the leaves in autumn, from green to brown or brown. It is a chemical change because the resulting products differ from the starting substances. Likewise, these reactions are accompanied by energy changes.įor example, sodium reacts with water to produce sodium hydroxide and hydrogen During this reaction, such amount of energy is released that the produced hydrogen gas is immediately burned in the air. The same amount of particles that existed before the reaction will continue to exist after this reaction. This means that during a chemical change the matter is not destroyed, but is transformed. When a chemical reaction occurs, the atoms of the involved substances reorganize.
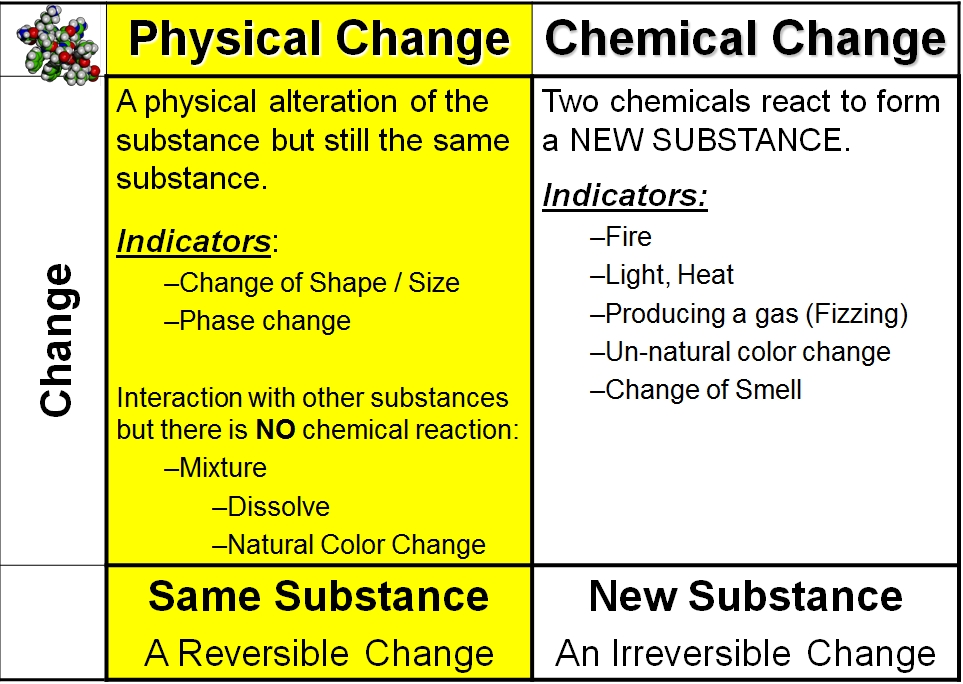

Others require heat so that they can be given, and are called endothermic. Some reactions produce heat These are known as exothermic.

The two processes mentioned above are called chemical reactions and, as a rule, are not reversible unless other chemical reactions are performed.


 0 kommentar(er)
0 kommentar(er)
